Table of Contents
When Joseph Priestley discovered oxygen in 1774, he answered age-old questions of why and how things burn. An Englishman by birth, Priestley was deeply involved in politics, religion, and science. Discovery and History of Oxygen.
However, when his vocal support for the American and French revolutions made remaining in his homeland dangerous, Priestley left England in 1794 and continued his work in America until his death.
Oxygen
A nonmetallic chemical element of Group 16 (VIa, or the oxygen group) of the periodic table. Oxygen cylinder bd is a colorless, odorless, tasteless gas essential to living organisms, being taken up by animals, which convert it to carbon dioxide plants, in turn, utilize carbon dioxide as a source of carbon and return the oxygen to the atmosphere.
Oxygen forms compounds by reaction with practically any other element and by reactions that displace elements from their combinations. In many cases, these processes are accompanied by the evolution of heat and light and, in such cases, are called combustions. Its most important compound is water.
With the careful laboratory work of Lavoisier, involving weighing and measuring, He discovered the air not to be a single element. Still, it was found to be composed primarily of two gases, one of which was oxygen, in a proportion of about 20%.
Priestley published that in a sealed chamber, this “pure form of air” (later named oxygen by Lavoisier) kept the mouse alive in his experiment.
The work of Priestley and Lavoisier disproved and overthrew the Phlogiston Theory, which speculated that a substance “phlogiston” was present in the air, which after combustion, became dephlogisticated. Discovery and History of Oxygen
The discovery of oxygen as an element and its roles in animal respiration and combustion were seminal events in the late 1700s when chemistry transitioned from a predominantly “black art” (alchemy) to science. Thus, in addition to being essential to life (certain bacteria are exceptions), oxygen had a significant role in the development of modern chemistry.
Oxygen from Minerals
In 1772, Carl Wilhelm Scheele discovered that red-hot manganese oxide produces a gas. He called the gas “fire air” because of the brilliant sparks it produced when it came in contact with hot charcoal dust.
He repeated this experiment by heating potassium nitrate, mercury oxide. And many other materials and produced the same gas. Next, he collected the gas in pure form using a small bag.
Finally, he explained the properties of “fire air” using the phlogiston theory, which Lavoisier soon discredited. He carefully recorded his experiments in notes but waited several years before publishing them.
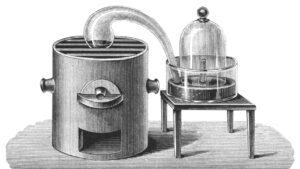
In 1774, Priestley repeated Scheele’s experiments using a 12-inch-wide glass “burning lens”; he focused sunlight on a lump of reddish mercuric oxide in an inverted glass container.
The gas emitted, he found, was five or six times as good as standard air. In succeeding tests, it caused a flame to burn intensely and kept a mouse alive about four times as long as a similar quantity of air.
Repeat Scheele’s and Priestley’s experiments:
Heating materials and breathing vapors could be hazardous. Therefore, as a rule: this experiment should be performed under the supervision of teachers or adults familiar with safety procedures.
Consult your teacher or other knowledgeable adults and experts about how to obtain the mentioned materials. And use them properly and safely in this experiment. Do not do this experiment alone! Discovery and History of Oxygen
Understanding the Composition of Air
In the mid-18th century, the concept of an element was still evolving. Researchers had distinguished no more than two dozen elements, depending on who was counting.
Moreover, it wasn’t straightforward how air fit into that system. Nobody knew what it was. And researchers kept finding that He could convert it into various forms that they routinely spoke of as different “airs.”
The principal method for altering the nature of air, early chemists learned, was to heat or burn some compound in it. The second half of the 1700s witnessed an explosion of interest in such gases. The steam engine was transforming civilization. And scientists of all types were fascinated with combustion and the role of air in it.
Occurrence and properties
At 46 percent of the mass, oxygen is the most abundant element in Earth’s crust. The proportion of oxygen by volume in the atmosphere is 21 percent and by weight in seawater is 89 percent. In rocks, it is combined with metals. And nonmetals in the form of acidic oxides such as sulfur, carbon, aluminum, and phosphorus.
Or basic such as those of calcium, magnesium, and iron. And salt like compounds that may be regarded as formed from the acidic. And basic oxides, such as sulfates, carbonates, silicates, aluminates, and phosphates.
Plentiful as they are, these solid compounds are not helpful as sources of oxygen because separating the element from its tight combinations with the metal atoms is too expensive.
Commercial production and use
When required in tonnage quantities, oxygen is prepared by the fractional distillation of liquid air. Of the main components of air. Oxygen has the highest boiling point and therefore is less volatile than nitrogen and argon. In addition, the process takes advantage of the fact that it cools when compressed gas is allowed to expand.

